Promoting Vaccination in Maternity Wards - Motivational Interview Technique Reduces Hesitancy and Enhances Intention to Vaccinate, Results from a Multicentre Non-Controlled Pre- and Post-Intervention RCT-Nested Study, Quebec, March 2014 to February 2015
Centre de recherche du CHUS (Gagneur, Battista, Lemaitre, Farrands, Gosselin); Université de Sherbrooke (Gagneur, Battista, Sauvageau, Petit); Centre de recherche du Centre Hospitalier Universitaire de Québec (Boucher, Boulianne); Université de Montréal (Tapiero, Quach); McGill University Health Centre Research Institute - Vaccine Study Centre (Quach); Laval University (De Wals, Sauvageau); Institut national de santé publique du Québec (Boulianne, Sauvageau, Ouakki, Dubé); Institut universitaire de première ligne en santé et services sociaux du CIUSSS de l'Estrie - CHUS (Jacques)
"Many countries are grappling with growing numbers of parents who delay or refuse recommended vaccinations for their children."
Conducted as a regional pilot, monocentric, quasi-experimental study in the Eastern Townships region of the Province of Quebec, Canada from March 2010 to February 2011, PromoVac was a response to the fact that traditional educational methods (e.g., information pamphlets, communication interventions aiming to provide information) have proven inefficient in addressing vaccine hesitancy (VH). The vaccination promotion programme (see Related Summaries, below) was based on a face-to-face intervention with parents conducted postpartum in maternity wards; it was further refined to include a standardised information session and motivational-interview (MI) techniques. Detailed in this article, the PromoVaQ study aimed to scale up PromoVac to a Province-wide multicentric study, conducted in 4 university hospital maternity wards between March 2014 and February 2015, in order to measure how the MI-based postpartum intervention impacted post-intervention vaccine intention (VI) and VH in participant parents of newborns.
This study was a pragmatic, unblinded, parallel-randomised controlled trial (RCT) powered to compare the impact of the MI-based intervention to the standard of care provided to parents of 2-day-old newborns on the overall vaccine coverage for children aged 24 months. Local research assistants were trained to provide a standardised intervention, and a 2-week trial period was conducted at each maternity ward before the study launch. Lasting about 20 minutes, the MI-based intervention was administered individually to consenting parents 24-48 hours after delivery in their maternity ward room.
The rationale was to accompany parents, in a non-judgmental manner, from their own stage of VI to the next stage by tailoring the intervention accordingly. It is based on a patient-centred approach aimed at increasing parental motivation through exploring and solving personal inherent ambivalences towards immunisation of their infant. The main content covered 5 main areas: (i) vaccine-preventable diseases (VPDs) and their consequences, (ii) vaccines and their effectiveness, (iii) the importance of the immunisation calendar in infants, (iv) reluctance to vaccinate and vaccination side effects, and (v) vaccination services and facilities in each of the study regions.
The primary outcome was VI measured using a validated questionnaire based on the health belief model (HBM), where answers were provided according to a 4-category Likert scale (certainly not, probably not, probably, and certainly). The secondary outcome was parental VH measured using Opel's validated questionnaire. Of participants, 1,223 completed the question on VI pre- and post-intervention, and their results are thus the focus of this report.
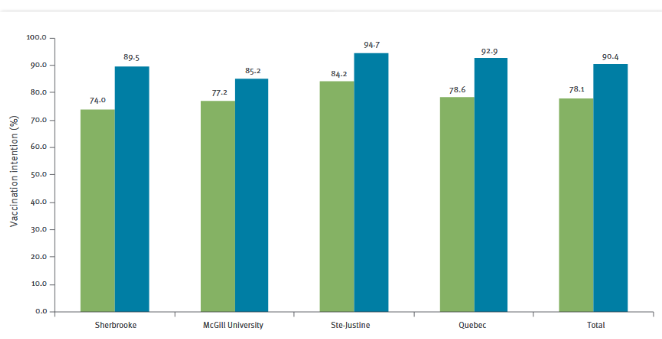
Prior to the intervention, total intention to "certainly" vaccinate their infant at 2 months of age was 78.1% among all participants combined and was significantly different between participating maternity wards (p=0.02). Following the intervention, the total intention to "certainly" vaccinate rose to 90.4%, a total 12% increase between pre- and post-intervention (p<0.0001). There was no significant proportion differences (post- vs pre-intervention) between the 4 study sites (p=0.24), suggesting that the effect of the intervention was comparable at each site. A significant rise in intention to "certainly" vaccinate was observed at each site post-intervention (p<0.0001) each site).
Participant VH significantly decreased post-intervention. Overall, the combined data from the 4 study sites showed that the relative proportion of participants with lowest VH (score 0-29) rose from 55.9% to 78.8% (41% increase), while those with intermediate and highest levels of VH (score 30-49 and over 50) decreased from 44.1% to 21.1%. Prior to the intervention, 15.6% of the overall population displayed high VH (over 50%). This fraction decreased to only 5.2% post-intervention (p<0.0001). The mean Opel score significantly decreased at each site between pre- and post-intervention evaluations (p<0.0001).
The pre-/post-impact of the intervention was found to be effective, irrespective of the potential confounding sociodemographic and cultural factors. In exploring the reasons behind its effectiveness, the researchers write: "We found that the MI-based intervention matched participant's expectations and needs and we believe this was attributable to the MI approach and techniques used in our intervention. For example, we facilitated a highly respectful and empathetic discussion of participants' concerns about childhood vaccination, which in turn, contributed to help build a trusting relationship between parents and research assistants. In addition, we ensured parents were given an opportunity to freely voice their concerns and questions about immunisation in the absence of any judgmental attitude from the healthcare professional."
The researchers note that several studies have shown that VI is correlated with the decision and behaviour to vaccinate. For example, a study on vaccination against influenza in Dutch healthcare personnel demonstrated that VI was a significant predictor of vaccination behaviour, with an odds ratio of 15.50 (95% confidence interval (CI): 9.24-25.99). Going forward, they plan to assess the impact of their MI-based intervention on child vaccine coverage at later ages and to correlate these with VI and VH scores.
Eurosurveillance. 2019;24(36):pii=1800641. https://doi.org/10.2807/1560-7917.ES.2019.24.36.1800641.
- Log in to post comments
